The Role of Hydrogen Fuel Cells in Heavy Duty Vehicles
Electrochemical Reactions: The Heart of the Process
At their core, fuel cells function as electrochemical devices, transforming the chemical energy stored in fuels—primarily hydrogen—directly into electrical energy. This transformation relies on a sequence of oxidation-reduction reactions, where hydrogen atoms undergo oxidation at the anode, releasing electrons. These electrons then travel through an external circuit, producing electricity, before reuniting with oxygen at the cathode to form water. Grasping the nuances of these electrochemical processes is essential for enhancing fuel cell efficiency and performance.
The effectiveness of these reactions depends heavily on factors such as the materials used for catalysts and the cell's overall design. While catalysts like platinum or platinum-based alloys accelerate these reactions, their steep cost poses a major obstacle to broader implementation. Discovering more affordable and efficient catalyst alternatives is pivotal for advancing fuel cell technology.
Types of Fuel Cells: Diverse Designs for Diverse Needs
Fuel cells come in various forms, each tailored to specific applications with unique benefits and drawbacks. Proton exchange membrane fuel cells (PEMFCs), for instance, are favored for portable uses due to their compact size and moderate operating temperatures. However, their reliance on pure hydrogen fuel can be a logistical hurdle.
Alkaline fuel cells (AFCs), which operate at elevated temperatures, can accommodate a broader range of fuels, including methanol. Solid oxide fuel cells (SOFCs) boast high efficiency but demand even higher temperatures, making them ideal for stationary power generation.
Catalyst Materials: Crucial for Efficiency
The performance and efficiency of fuel cells hinge on the catalyst materials embedded within them. These catalysts drive the electrochemical reactions at the anode and cathode, enabling the conversion of chemical energy into electricity. Advancements in catalyst research are vital for boosting efficiency and cutting costs.
Ongoing studies are exploring alternatives like transition metal oxides and non-precious metals, which could make fuel cell technology more sustainable and widely accessible.
Electrolyte Materials: The Conduit for Ion Flow
The electrolyte in a fuel cell serves as the pathway for ion movement between the anode and cathode. Different fuel cell types employ distinct electrolyte materials, each influencing the cell's operating temperature, performance, and cost.
Hydrogen Production: A Crucial Component
A key challenge in hydrogen fuel cell technology is the production of hydrogen itself. Current methods, such as steam methane reforming, often depend on fossil fuels, which can offset the environmental advantages of fuel cells. Developing renewable hydrogen production techniques is critical for realizing the full potential of this technology.
Innovative approaches, like electrolysis powered by solar or wind energy, could sever the link between hydrogen production and fossil fuels, paving the way for a truly sustainable energy ecosystem.
Power Density and Efficiency: Key Performance Metrics
Power density and efficiency are pivotal metrics for evaluating fuel cell performance. Higher power density enables greater energy output in compact designs, ideal for portable applications. Efficiency, meanwhile, measures the electrical energy generated relative to the chemical energy input. Optimizing these factors is essential for broader adoption.
Fuel Cell Applications: From Transportation to Power Generation
Fuel cell technology is versatile, spanning multiple industries as a cleaner alternative to conventional energy sources. In transportation, fuel cells are being tested for vehicles, offering a potential solution to curb emissions. For stationary power, they provide reliable, eco-friendly electricity for homes and businesses.
Beyond these uses, fuel cells are also being explored for portable electronics, backup power systems, and industrial processes. Continued innovation is key to expanding their applications and improving their capabilities.
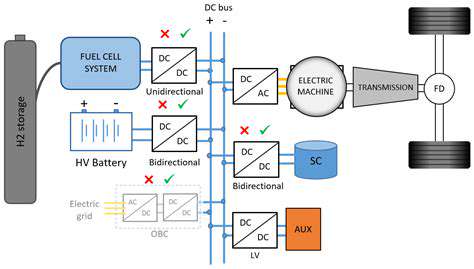
The Future Outlook: Hydrogen Fuel Cells and the Road Ahead

Hydrogen's Potential for Transportation
Transportation is a major source of greenhouse gas emissions, and hydrogen fuel presents a compelling alternative to fossil fuels. Hydrogen-powered vehicles could revolutionize the industry by slashing emissions and fostering a cleaner planet. This potential stems from fuel cells' ability to convert hydrogen and oxygen directly into electricity, emitting only water as a byproduct. This zero-emission feature makes hydrogen an attractive option for phasing out traditional combustion engines.
Moreover, the infrastructure for hydrogen vehicles is steadily expanding. As more fueling stations are built and technology evolves, hydrogen could play an increasingly vital role in reducing our reliance on finite fossil fuels.
Challenges and Considerations
Despite its promise, hydrogen fuel faces significant adoption barriers. High production and storage costs are major obstacles, and current methods often rely on fossil fuels, undermining environmental benefits. Developing sustainable, cost-effective hydrogen production techniques is essential for long-term success.
Another hurdle is the need for extensive infrastructure, including production facilities, storage systems, and fueling stations. While this requires substantial investment, it is indispensable for unlocking hydrogen's full potential.
Production and Sustainability
Hydrogen production methods are a critical factor in its sustainability. Current fossil fuel-based approaches detract from its environmental advantages. Renewable methods, such as electrolysis powered by solar or wind energy, offer a greener alternative. Electrolysis splits water into hydrogen and oxygen, providing a pathway to a sustainable hydrogen economy.
Additionally, the entire hydrogen lifecycle—from production to usage—must be scrutinized for environmental impact. Prioritizing renewable energy sources for hydrogen production can minimize its ecological footprint.
Economic Implications and Policy
Shifting to a hydrogen economy carries significant economic ramifications. Investments in research, infrastructure, and development can spur job creation and industry growth. The evolution of fuel cell technology and manufacturing processes may also drive innovation and economic expansion.
Government policies are instrumental in accelerating this transition. Incentives for hydrogen technology development and deployment, coupled with regulations ensuring sustainability, can hasten the advent of a hydrogen-powered future.
Read more about The Role of Hydrogen Fuel Cells in Heavy Duty Vehicles
Hot Recommendations
- Offshore Wind for Industrial Power
- Agrivoltaics: Dual Land Use with Solar Energy Advancements: Sustainable Farming
- Hydrogen as an Energy Storage Medium: Production, Conversion, and Usage
- Utility Scale Battery Storage: Successful Project Case Studies
- The Role of Energy Storage in Grid Peak Shaving
- The Role of Startups in Renewable Energy
- The Role of Blockchain in Decentralization of Energy Generation
- The Future of Wind Energy Advancements in Design
- Synchronous Condensers and Grid Inertia in a Renewable Energy Grid
- Corporate Renewable Procurement for Government Agencies