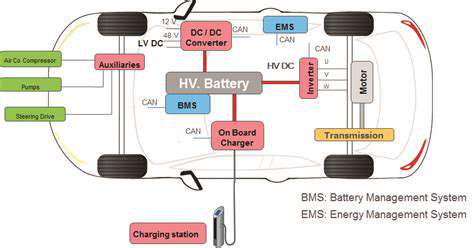
Impact of High Power Charging on EV Battery Life
Every battery, whether powering a smartphone or an electric grid, inevitably loses performance over time. This phenomenon, known as battery degradation, stems from intricate chemical and physical transformations occurring during repeated charge-discharge cycles. What many don't realize is that these changes aren't just surface-level - they fundamentally alter the battery's molecular structure. By examining these processes closely, engineers can develop more resilient energy storage solutions.
Multiple elements accelerate battery wear, from internal electrochemical reactions to environmental influences like heat and humidity. These factors collectively diminish capacity, boost internal resistance, and shorten operational lifespan. Interestingly, even when unused, batteries undergo gradual self-discharge that contributes to long-term degradation. This explains why device manufacturers emphasize proper storage conditions for optimal battery preservation.
Electrochemical Reactions and Degradation Mechanisms
At the heart of every battery's operation - and its eventual decline - lie complex electrochemical processes. During normal use, electrodes experience continuous oxidation and reduction, forming protective solid electrolyte interphase (SEI) layers. While initially beneficial, these layers can grow unstable over hundreds of cycles, gradually consuming active materials and reducing energy storage capacity.
Perhaps the most visually striking degradation phenomenon involves dendritic growth - microscopic metallic filaments that resemble frost patterns on a windowpane. These delicate structures pose serious risks, potentially breaching separators and creating hazardous short circuits. Simultaneously, lithium ions face increasing difficulty moving through the battery's architecture, much like traffic congestion worsening in an aging city's road network.
The cumulative effect of these processes resembles geological erosion - electrode materials slowly fracture and crumble, reducing available surface area for energy exchange. This explains why older batteries not only hold less charge but also deliver power less efficiently. Modern research focuses on developing self-healing materials that could counteract these destructive mechanisms.
External Factors Affecting Battery Life
Environmental conditions dramatically influence battery longevity in ways that often surprise consumers. Temperature extremes prove particularly damaging - excessive heat accelerates chemical breakdown like spoiling food, while extreme cold thickens electrolytes, hindering ion movement. Studies show that batteries stored at 25°C maintain significantly more capacity after five years than those exposed to 40°C environments.
Humidity and airborne contaminants present another often-overlooked threat. Moisture can corrode contacts and internal components, while industrial pollutants may chemically interact with battery materials. Proper storage becomes especially crucial for backup power systems that might sit unused for extended periods.
Manufacturing imperfections create localized stress points that become failure hotspots over time. Imagine microscopic weak spots in a bridge that eventually develop into cracks under constant traffic. This underscores why reputable battery manufacturers invest heavily in quality control and material purity verification throughout production.
Long-Term Battery Health and Cycle Life
Understanding Battery Degradation
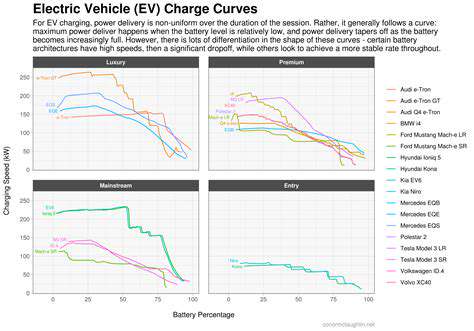
Fast charging technology presents a classic trade-off between convenience and longevity. The physics behind this dilemma involves intense ion traffic jams during rapid energy transfers, causing structural stress comparable to repeatedly stretching and compressing a rubber band to its limits. Different battery formulations handle this stress with varying success - lithium iron phosphate (LFP) chemistries generally withstand fast charging better than conventional lithium-ion variants.
Charging parameters create complex interactions affecting degradation rates. For instance, charging an already warm battery at high rates compounds stress, while moderate temperatures with controlled current profiles can minimize damage. Depth of discharge also plays a crucial role - shallower cycles generally prove less taxing than full 0-100% charges.
Impact on Cycle Life
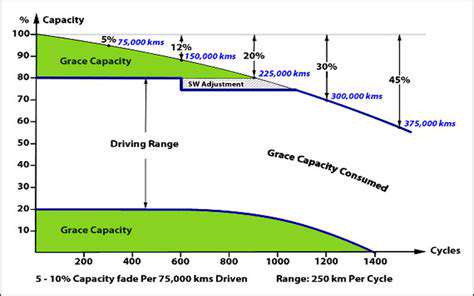
Cycle life represents perhaps the most tangible metric for battery longevity, indicating how many full charges a battery can endure before significant capacity loss. Think of each fast-charging session as sprinting instead of walking - while occasionally fine, making it a habit inevitably leads to earlier exhaustion. The cumulative microscopic damage from aggressive charging manifests as reduced range and performance, particularly noticeable in electric vehicles after several years of use.
This degradation follows predictable patterns - initially rapid capacity loss stabilizes into gradual decline before accelerating again as end-of-life approaches. Real-world data shows that batteries subjected to frequent fast charging may reach 80% original capacity hundreds of cycles sooner than those charged more gently. For EV owners, this translates directly to resale value and potential replacement costs.
Strategies for Maintaining Battery Health
Smart charging habits can significantly extend battery service life without sacrificing convenience. Consider these practical approaches:
- Reserve fast charging for when truly needed, using standard charging for routine top-ups
- Maintain charge levels between 20-80% for daily use, saving full charges for long trips
- Allow battery cooling after fast charging before immediately discharging at high rates
Modern battery management systems provide valuable insights through diagnostic data. Monitoring voltage consistency across cells and tracking capacity trends helps identify potential issues early. Seasonal adjustments matter too - reducing charge speeds in extreme temperatures and avoiding leaving batteries at full charge in hot environments. These practices, combined with manufacturer-recommended maintenance, can help batteries deliver years of reliable service.