Comparing Energy Density of Next Gen Battery Technologies
Solid-State Batteries: A Revolution in Energy Storage
Solid-state batteries represent a significant advancement in energy storage technology, promising a paradigm shift from the current lithium-ion battery paradigm. These batteries leverage solid electrolytes, replacing the liquid or polymer electrolytes found in conventional lithium-ion batteries. This fundamental change offers substantial advantages, including enhanced safety, higher energy density, and potentially improved lifespan.
The key differentiator lies in the solid electrolyte, which eliminates the flammability risks associated with liquid electrolytes. This safety aspect is crucial for applications demanding reliable and safe energy storage, such as electric vehicles and portable electronics.
Enhanced Energy Density: A Crucial Advantage
One of the most compelling aspects of solid-state batteries is their potential for significantly higher energy density. This increased energy density translates directly into improved performance for electric vehicles, enabling longer ranges and faster charging times. Researchers are actively exploring various solid electrolyte materials to achieve this target, and early results are promising, suggesting substantial advancements in battery technology.
Improved Safety Profile: A Major Breakthrough
The inherent safety of solid-state batteries is a major advantage over their liquid electrolyte counterparts. The elimination of flammable liquid electrolytes minimizes the risk of thermal runaway, a critical safety concern in lithium-ion batteries. This safety aspect is essential for applications in electric vehicles and portable electronics, where safety is paramount.
Material Science Challenges: The Path Forward
While the potential of solid-state batteries is undeniable, significant material science challenges remain. Developing stable, high-ionic conductivity solid electrolytes with compatible electrodes is crucial for achieving practical battery performance. Researchers are actively investigating various materials, including ceramic oxides, polymers, and glasses, to address these challenges and unlock the full potential of this technology.
Challenges in Manufacturing: Scaling Up Production
Beyond material science, the manufacturing process for solid-state batteries is also a significant hurdle. Scaling up production to meet the demands of the growing market for electric vehicles and other applications requires innovative manufacturing techniques and a substantial investment in research and development. Overcoming these challenges is essential for making solid-state batteries a viable and commercially successful technology.
Cost Considerations: Bridging the Gap to Commercialization
The high cost of solid-state battery materials and manufacturing processes is a significant obstacle to widespread adoption. Reducing these costs is crucial for achieving commercial viability. Extensive research and development efforts are focused on lowering material costs and optimizing manufacturing processes to make solid-state batteries more affordable for consumers and businesses.
Comparing to Other Next-Gen Batteries: A Holistic View
Solid-state batteries are not the only next-generation battery technology being explored. Other contenders, such as lithium-sulfur batteries and lithium-air batteries, also hold promise. A comprehensive comparison of energy densities, safety profiles, and manufacturing challenges is essential to understanding the relative merits of each technology and identifying the most promising path forward for advanced energy storage.
Lithium-Sulfur Batteries: High Energy Density Potential
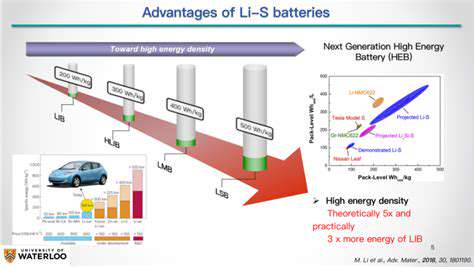
Lithium-Sulfur Batteries: A Promising Alternative
Lithium-sulfur (Li-S) batteries represent a promising alternative to conventional lithium-ion batteries due to their exceptionally high theoretical energy density. This significant advantage stems from the high abundance and low cost of sulfur, a crucial component of these batteries. Leveraging sulfur's abundance offers a potential pathway to more affordable and sustainable energy storage solutions.
Chemistry and Mechanism
The fundamental chemistry behind Li-S batteries involves the conversion of sulfur into lithium polysulfides. These polysulfides then migrate to the cathode, where they undergo further reactions. This complex electrochemical process is still under active investigation, and a complete understanding of its intricacies is crucial for optimizing battery performance.
Challenges in Lithium-Sulfur Batteries
Despite their impressive potential, Li-S batteries face significant hurdles. One of the primary challenges is the shuttle effect, where lithium polysulfides dissolve in the electrolyte and diffuse to the anode, leading to capacity fading and reduced battery lifespan. Addressing this crucial issue through electrolyte engineering and cathode design is a critical area of research.
Electrolyte Optimization
The electrolyte plays a critical role in the performance of Li-S batteries. Developing stable and effective electrolytes that can effectively prevent the dissolution of lithium polysulfides is essential for improving cycling performance and overall battery life. Researchers are exploring various approaches, such as polymer-based electrolytes and ionic liquid electrolytes, to achieve this goal.
Cathode Materials and Design
Cathode materials are another key component in Li-S batteries. The design and engineering of efficient cathode materials that can effectively trap and react with lithium polysulfides are essential for preventing the shuttle effect. Novel nanostructured materials and composite designs are being explored to enhance the interaction between sulfur and lithium.
Anode Materials and Design
The anode material, typically lithium metal, also plays a crucial role. Maintaining the stability of the lithium metal anode is essential, as it tends to undergo side reactions which can negatively impact battery performance. Researchers are exploring different anode coatings and designs to mitigate these issues and ensure long-term battery stability.
Current Research and Future Directions
Ongoing research in Li-S battery technology focuses on enhancing the cycle life, rate capability, and safety of these batteries. Significant advancements are being made in materials science, electrolyte development, and cell design. Future directions include the development of advanced characterization techniques and improved understanding of the complex electrochemical processes involved in Li-S batteries.
Lithium-Air Batteries: A Revolutionary Approach
Lithium-Air Battery Fundamentals
Lithium-air batteries represent a promising avenue for achieving significantly higher energy densities compared to conventional lithium-ion batteries. The core concept revolves around utilizing atmospheric oxygen as the cathode, a substantially lighter and more readily available component than the complex, often expensive materials employed in traditional lithium-ion battery cathodes. This inherent advantage in material availability, combined with the high theoretical energy density of lithium-air systems, makes them a fascinating area of research.
Fundamentally, the process involves lithium metal reacting with oxygen to form lithium oxide, generating a significant amount of energy in the process. This electrochemical reaction is the driving force behind the battery's operation, offering the potential for substantial improvements in energy storage capacity.
Challenges in Lithium-Air Battery Technology
Despite the compelling theoretical advantages, lithium-air batteries face several significant hurdles. One major challenge lies in the inherent instability of the lithium-air reaction. The formation of solid-electrolyte interphases (SEIs) during the charging and discharging cycles is complex and often leads to capacity fading, a crucial factor affecting the overall performance of the battery.
Furthermore, the need for efficient oxygen reduction and evolution reactions at the cathode poses substantial challenges for catalyst design and material selection. Finding durable and cost-effective catalysts that can facilitate these reactions at high rates and maintain performance over extended periods is a critical area of ongoing research and development.
Energy Density and Specific Capacity
The high theoretical energy density of lithium-air batteries stems from the abundance of oxygen in the atmosphere. This makes them a potential game-changer for applications demanding substantial energy storage, such as electric vehicles, portable electronics, and grid-scale energy storage. The specific capacity, a crucial metric, is a measure of the amount of energy that can be stored per unit mass of the battery. Lithium-air batteries exhibit a significantly higher specific capacity compared to lithium-ion batteries, demonstrating the potential for greater energy storage in a smaller package.
Catalyst Development for Enhanced Performance
Effective catalysts are essential for enabling efficient oxygen reduction and evolution reactions at the cathode. Various materials, including transition metals and their oxides, are being investigated for their catalytic properties. Optimizing these catalysts to enhance activity, stability, and cost-effectiveness is a critical focus of research in this field. The development of novel catalyst designs is essential to overcome the limitations of conventional catalysts and achieve optimal performance in lithium-air batteries.
Electrolyte and Separator Materials
Selecting appropriate electrolytes and separators is crucial for the long-term stability and safety of lithium-air batteries. The high reactivity of lithium and oxygen necessitates the development of robust electrolytes that can prevent unwanted side reactions and maintain ionic conductivity throughout the battery's lifecycle. Moreover, suitable separators are required to prevent short circuits and maintain the structural integrity of the battery during operation. The development of robust, yet cost-effective, electrolyte and separator materials is critical for successful commercialization.
Safety Considerations and Practical Applications
Lithium-air batteries, despite their potential, pose safety concerns due to the inherent reactivity of lithium and oxygen. Careful design and engineering are necessary to mitigate these risks and ensure the safe operation of these batteries. Addressing safety concerns alongside performance optimization is essential for their widespread adoption. Potential practical applications include electric vehicles, portable electronics, and even large-scale energy storage systems. The success of lithium-air batteries will hinge on overcoming the significant technological challenges and demonstrating their safety and reliability in real-world applications.
Read more about Comparing Energy Density of Next Gen Battery Technologies
Hot Recommendations
- Offshore Wind for Industrial Power
- Agrivoltaics: Dual Land Use with Solar Energy Advancements: Sustainable Farming
- Hydrogen as an Energy Storage Medium: Production, Conversion, and Usage
- Utility Scale Battery Storage: Successful Project Case Studies
- The Role of Energy Storage in Grid Peak Shaving
- The Role of Startups in Renewable Energy
- The Role of Blockchain in Decentralization of Energy Generation
- The Future of Wind Energy Advancements in Design
- Synchronous Condensers and Grid Inertia in a Renewable Energy Grid
- Corporate Renewable Procurement for Government Agencies